The InBios COVID-19 rapid test is a user-friendly, FDA-authorized diagnostic tool designed for quick detection of SARS-CoV-2 antigens․ It provides results in 20 minutes with high accuracy, making it ideal for home use․ Follow the instructions carefully to ensure reliable outcomes and safe testing experience․
1․1 Overview of the InBios COVID-19 Rapid Test
The InBios COVID-19 Rapid Test is an FDA-authorized antigen test designed for quick detection of SARS-CoV-2․ It is user-friendly, providing results in 20 minutes, and is suitable for both healthcare settings and home use․ The test uses a nasal swab sample and includes a test strip for easy interpretation․ Its simplicity and accuracy make it a reliable tool for rapid COVID-19 screening, ensuring timely detection and response․
1․2 Importance of Rapid Testing in COVID-19 Pandemic
Rapid testing is crucial in the COVID-19 pandemic for early detection and containment․ The InBios COVID-19 Rapid Test enables quick identification of infections, reducing transmission risks and allowing timely isolation․ Its accessibility ensures widespread testing, supporting public health strategies and individual safety․ Early results facilitate faster decision-making, making rapid tests like InBios essential for controlling outbreaks and protecting vulnerable populations effectively․
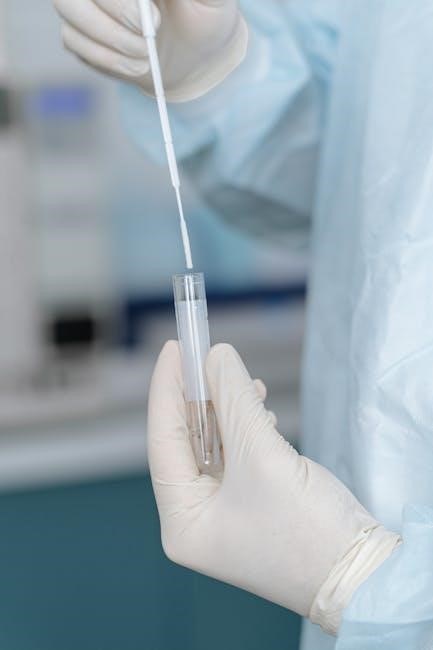
Preparation for the Test
Prepare by gathering all materials, reading instructions, and ensuring a clean environment․ Visit www․inbios․com/covid-19 for detailed guidance to ensure accurate and safe testing․
2․1 Gathering Required Materials
To begin, collect the InBios COVID-19 rapid test kit, which includes a test strip, sample collection swab, and dropper․ Ensure all components are intact and within the expiration date․ Additionally, have a clean, flat surface and good lighting available․ Properly washing your hands before starting is crucial for accurate results and to prevent contamination․
2․2 Understanding the Test Components
The InBios COVID-19 rapid test kit includes a test strip, nasal swab, dropper, and instructions․ The test strip detects SARS-CoV-2 antigens, while the swab collects the nasal sample․ The dropper transfers the sample to the strip․ Ensure all components are sterile and within their expiration dates․ Properly handling each part is essential for accurate results and to avoid contamination during the testing process․
2․3 Preparing the Testing Environment
Before starting, choose a clean, flat surface for the test kit․ Ensure the area is well-lit and free from distractions․ Wash your hands thoroughly with soap and water․ Remove any bulky jewelry from your hands to handle the components easily․ Keep the test kit and its contents away from direct sunlight and moisture to maintain their integrity․ A prepared environment ensures smooth testing and accurate results․

Step-by-Step Testing Instructions
Handle all components carefully, ensure proper specimen collection, and follow each step-by-step instruction for quick and accurate results in a clean, stable environment․
3․1 Opening the Test Kit Safely
Begin by washing your hands thoroughly․ Carefully open the outer packaging, ensuring no damage to the components inside․ Inspect the test kit for any visible defects or tampering․ Once confirmed intact, gently remove the test strip and other materials, taking note of their proper arrangement․ Wear gloves if available to minimize direct contact and avoid contamination․ Proceed with caution to maintain sterility and accuracy․
3․2 Collecting the Nasal Swab Sample
Gently insert the swab into one nostril, guiding it straight back until resistance is met․ Rotate the swab gently for 10-15 seconds to collect a sample․ Repeat in the other nostril using the same swab․ Avoid touching the swab tip to any surfaces․ After collection, carefully remove the swab and proceed to the next step without delay․ Handle the swab carefully to prevent accidental contamination․
3․3 Adding the Sample to the Test Strip
Dip the swab in the provided buffer, ensuring the sample is evenly distributed․ Gently apply 2-3 drops to the test strip’s designated area, aligning the swab with the strip’s edge․ Avoid touching the reactive area․ Wait 10-15 minutes for the control (C) and test (T) lines to appear․ Dispose of the swab in a biohazard bag and wash hands․ Follow instructions carefully to avoid errors and ensure accurate results․
3․4 Waiting for the Results
After adding the sample, wait 10-15 minutes for the results․ Avoid touching the test strip or rechecking it․ The control line (C) should appear first, indicating the test is working․ If the test line (T) appears, the result is positive․ No lines or a faint control line means an invalid test․ Results are final and cannot be reinterpreted after the specified timeframe․ Ensure accuracy by adhering to the instructions provided․

Interpreting the Results
Ensure the control line (C) appears for a valid test․ A positive result shows a test line (T)․ No lines or a missing control line indicates an invalid test․
4․1 Understanding Positive, Negative, and Invalid Results
A positive result shows a visible test line, indicating the presence of COVID-19 antigens․ A negative result displays no test line, suggesting no infection․ If the control line is missing or unclear, the test is invalid․ Invalid results may occur due to improper testing technique or kit damage․ Always repeat the test with a new kit if results are unclear or invalid․
4․2 What to Do If the Result is Positive
If your InBios COVID test result is positive, isolate immediately to prevent spreading the virus․ Notify close contacts and consult a healthcare provider for further guidance․ Follow public health recommendations, including wearing a mask and maintaining distance․ Consider additional testing if recommended and adhere to all safety measures to protect yourself and others․
4․3 What to Do If the Result is Negative
If your InBios COVID test result is negative, continue following public health guidelines, such as wearing a mask and maintaining social distancing․ Monitor for symptoms and seek medical advice if they develop․ Even with a negative result, additional testing may be recommended depending on exposure history and symptoms․ Always follow healthcare provider instructions for further actions․
Post-Test Procedures
Dispose of the test kit safely, report results to healthcare providers, and follow up with additional testing if recommended․ Ensure all steps align with guidelines provided․
5․1 Proper Disposal of the Test Kit
After completing the test, dispose of the InBios COVID test kit responsibly․ Wear gloves and a mask, then seal all components in a plastic bag․ Dispose of the bag in your regular trash․ Avoid sending the kit to special collection centers․ Wash your hands thoroughly after disposal․ Follow local regulations for biomedical waste to ensure safety and prevent contamination․
5․2 Reporting the Results to Healthcare Providers
After obtaining your test results, immediately inform your healthcare provider, especially if positive․ Share details like the date of the test and any symptoms․ This ensures accurate records and appropriate guidance․ For negative results, still consult your provider if symptoms persist or worsen․ Reporting is crucial for tracking and managing COVID-19 cases effectively․
5․3 Following Up with Additional Testing if Needed
If your InBios COVID-19 test result is positive or inconclusive, consult your healthcare provider for further evaluation․ They may recommend a molecular test for confirmation․ Additional testing is crucial for individuals with symptoms or high-risk exposure․ Ensure to follow your provider’s guidance for any required follow-up testing to confirm results and manage your health effectively․

Safety Precautions
Handle the InBios COVID test kit with care, wearing gloves to prevent contamination․ Follow all safety guidelines to ensure accurate results and protect yourself from potential exposure․
6․1 Handling the Test Kit and Components
Always handle the InBios COVID test kit and its components with clean, dry hands or gloves to avoid contamination․ Ensure all parts are stored in a dry place at room temperature․ Avoid touching the test strip or swab tip to prevent inaccurate results․ Follow the instructions carefully to maintain the integrity of the test components and ensure reliable outcomes․
6․2 Avoiding Cross-Contamination
To prevent cross-contamination, use clean gloves and ensure all surfaces are disinfected․ Handle each test component separately and avoid touching the swab tip or test strip․ Do not reuse any parts of the kit․ Keep the test area clean and store unused components in their original packaging․ Properly dispose of used materials to maintain test accuracy and safety․
6․3 Storing the Test Kit Properly
Store the InBios COVID-19 test kit in a cool, dry place between 2-30°C (36-86°F)․ Avoid exposure to direct sunlight, moisture, or extreme temperatures․ Do not open the kit until ready to use․ Keep it out of reach of children and pets․ Ensure all components remain sealed in their original packaging to maintain integrity and accuracy until the test is performed․
Common Mistakes to Avoid
Common mistakes include improper swabbing technique, not following instructions, and misinterpreting results․ Avoid these errors to ensure accurate and reliable test outcomes․
7․1 Incorrect Swabbing Technique
Incorrect swabbing technique is a common mistake that can lead to inaccurate results․ Ensure the swab is inserted properly into both nostrils, following the instructions carefully․ Avoid shallow swabs or missing the designated area, as this can result in a false negative․ Proper technique ensures sufficient sample collection for accurate detection of SARS-CoV-2 antigens․
7․2 Not Following the Instructions Closely
Not following the instructions carefully can lead to inaccurate results or test failure․ Deviating from the step-by-step guide, such as skipping waiting times or mishandling components, can compromise accuracy․ Always read and adhere to the provided instructions to ensure reliable outcomes and maintain the integrity of the testing process for effective COVID-19 detection․
7․3 Misinterpreting the Results
Misinterpreting the results can lead to incorrect conclusions about infection status․ Ensure to carefully observe the test strip for the control and test lines․ A faint line still indicates a positive result, while no line suggests a negative․ Always refer to the provided guidelines to avoid confusion and ensure accurate interpretation of the InBios COVID test outcomes․
Frequently Asked Questions
- Can the InBios COVID test be used for children?
- How accurate is the InBios COVID test?
- Can the test be used multiple times?
8․1 Can the Test be Used for Children?
The InBios COVID-19 rapid test can be used for children, but it is recommended for individuals aged 2 and older․ For younger children, adult assistance is required for swab collection․ Ensure proper technique to avoid discomfort and ensure accuracy․ Always consult a healthcare provider for guidance on testing children․
8․2 How Accurate is the InBios COVID Test?
The InBios COVID-19 rapid test demonstrates high accuracy, with sensitivity and specificity rates exceeding 95%․ It reliably detects SARS-CoV-2 antigens, providing quick and trustworthy results․ However, accuracy depends on proper sample collection and adherence to instructions․ For conclusive results, follow the testing procedure carefully and consider additional testing if symptoms persist or exposure risk is high․
8․3 Can the Test be Used Multiple Times?
The InBios COVID-19 rapid test is designed for single use only․ Reusing the test strip, swab, or other components can lead to contamination or inaccurate results․ Each test kit contains unique components for one-time testing to ensure accuracy and safety․ Proper disposal of used test materials is essential to maintain hygiene and prevent cross-contamination․
Effective and easy to use, the InBios COVID-19 rapid test provides accurate results in minutes․ Always follow instructions and take proper precautions to ensure safety and reliability․
9․1 Summary of Key Points
The InBios COVID-19 rapid test is an FDA-authorized, easy-to-use diagnostic tool for detecting SARS-CoV-2 antigens․ It delivers accurate results in 20 minutes, making it ideal for at-home testing․ Proper nasal swab collection and adherence to instructions are crucial for reliable outcomes․ The test is designed for simplicity, ensuring safety and efficiency․ Always follow disposal guidelines and consult healthcare providers for further guidance if needed․
9․2 Final Tips for Effective Testing
For accurate results, always follow the InBios COVID-19 test instructions carefully․ Ensure proper swab technique and avoid cross-contamination․ Read results within the specified time frame (20 minutes)․ Store the test kit in a cool, dry place․ If positive, isolate immediately and consult a healthcare provider․ Regular testing helps in early detection and prevention of spread․ Stay informed and adhere to safety guidelines for reliable outcomes․
